Covid-19 Vaccine Pharmacovigilance report
This report was prepared by the World Council for Health (WCH). The report was prepared to determine whether sufficient pharmacovigilance data exists on WHO VigiAccess, CDC VAERS, EudraVigilance, and UK Yellow Card Scheme to establish a safety signal on Covid-19 vaccines.
These databases are not normally used to establish the safety of an intervention. However, Covid-19 vaccines are in Phase 3 trials, and their safety and efficacy have not yet been established. The majority of those who have received the intervention (several billion people) are not being monitored by the trials. In this report, the WCH aims to use these established pharmacovigilance databases to detect if there is a concerning safety signal in those not being monitored by the clinical trials.
This report collates pharmacovigilance data about Covid-19 vaccines and other commonly administered interventions from these databases. It collates data about the types of adverse event reports linked to Covid-19 vaccines on these databases. Using data from VAERS, FAERS, and historical records, the report collates data about the rate of adverse events that have been sufficient for product recall in the past.
Purpose of Report
This report was prepared to determine if there is sufficient data on well-established, existing pharmacovigilance databases to establish a safety signal regarding Covid-19 vaccines.
Covid-19 Vaccine Development was Rushed
Covid-19 vaccine development under Operation Warp Speed was rushed. Traditionally, vaccine development takes 10 years or more before large-scale production and distribution to a wide population. With Covid-19 vaccines, the product moved from exploratory, pre-clinical trials to large-scale manufacturing in just 10 months.
Traditional Vaccine Development Timeline Compared to Potential Operation Warp Speed (OWS) Timeline
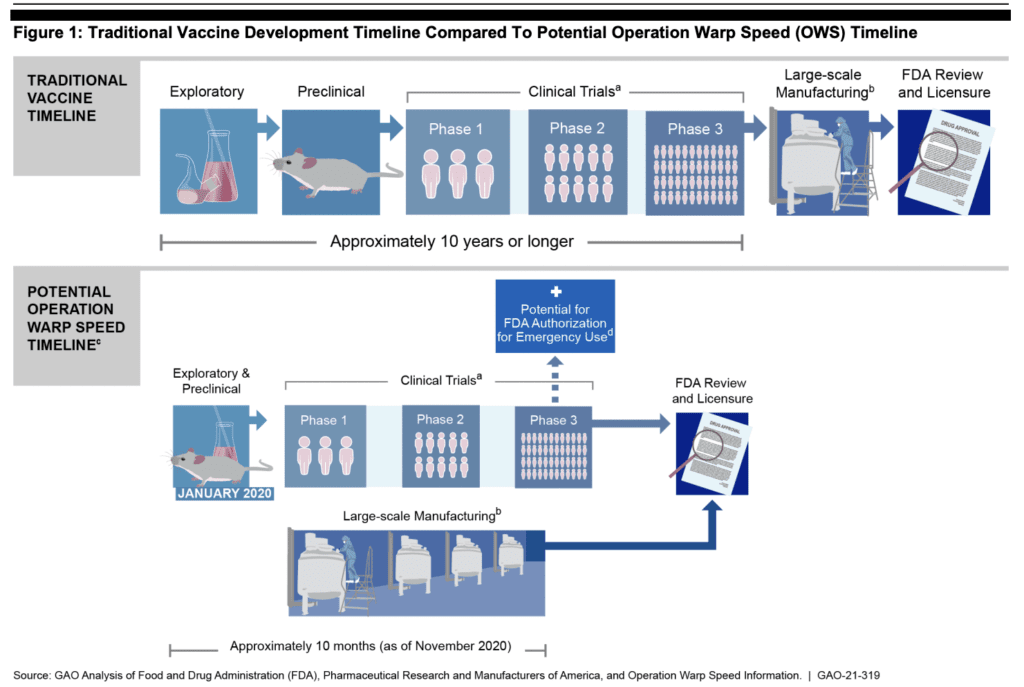
Because the development of these products was rushed, data about their safety are incomplete. In the interest of making a more complete picture of the safety of these novel products, this report collates:
1. Data from Pharmacovigilance Databases about Covid-19 Vaccines and Other Commonly Administered Vaccines and Interventions
This report collates adverse event data on COVID-19 vaccines from the following pharmacovigilance databases:
- The World Health Organization (WHO) – VigiAccess
- The US Center for Disease Control (CDC) – Vaccine Adverse Events Reporting System (VAERS)
- EudraVigilance – European Database of Suspected Adverse Drug Reaction Reports
- Medicines & Healthcare products Regulatory Agency – UK Yellow Card Reporting Site
The Covid-19 vaccine adverse event data gathered on each pharmacovigilance database is compared with the adverse event data of similar pharmacological products (other common vaccines) on the same databases when possible.
2. Data about the Types of Adverse Reactions Linked to Covid-19 Vaccines
In addition, this report examines the types of adverse reaction reports linked to Covid-19 vaccines on the above databases.
3. Data about the Rate of Adverse Events that is Sufficient for Product Recall
This report examines the parameters by which other vaccines and drugs have been recalled in the past.
This is an informational report created by World Council for Health to aid healthcare practitioners, scientists, and individual citizens in making informed decisions about Covid-19 vaccines.
The report seeks to answer the question: Are the pharmacovigilance data contained on these databases sufficient to establish a safety ‘signal’ for Covid-19 vaccines that indicates product recall?

Read The Report
Visit the World Council Of Health Website to see the full in-depth analysis of this report and help remain educated. Alternatively click the button below to download a PDF version of the report for free.






I’m on it with you. Unvaccinated and I’m so happy I stayed out.